Rémi Ouellette Blog #2
RESOLUTION OF RACEMIC MIXTURES
Hi guys! It's still Rémi! For this blog post, I decided to write about something different from engineering. In fact, in chemistry class, I recently learned about enantiomers and diastereomers. So, I decided to read more on the subject. I read an article on the resolution of racemic mixtures because my teacher taught us it was an annoying process, and I wanted to learn more about it.
Example of an enantiomer ((S)-2-hydroxypropanoic acid and (R)-2-hydroxypropanoic acid):

My source for this post:
Important organic chemistry vocabulary:
- Chiral carbon: A chiral carbon is a carbon atom linked with four different atoms or moieties. If a molecule contains a chiral atom, the molecule is said to be chiral. It will deviate polarized light.
- S or R configuration: Depending on the configuration of the atoms surrounding a chiral carbon, a molecule can be "S" or "R". To determine the molecule's configuration, the atoms or moieties surrounding the carbon atom must be analyzed and given a number from one to four. The number four will be given to the atom or moiety with the lowest number in the periodic table and the number one to the atom or moiety with the highest number. If they are placed clockwise when the number four atom or moiety is in the back, the carbon will be "R" and if not, it will be "S".
- Enantiomer: An enantiomer is the isomer of a chiral molecule with its carbon configuration reversed. For example, the enantiomer of an "S" molecule will be an "R" molecule just like the enantiomers of lactic acid in the image at the beginning of this blog post. Enantiomers share the same physical and chemical properties but have different biological effects.
- Racemic mixture: A racemic mixture contains fifty percent of one enantiomer and fifty percent of the other. A resolution is the process of separating a racemic mixture.
- Diastereomer: A diastereomer contains at least two chiral carbons. At least one of the chiral carbon atoms is configured the same way in both diastereomers but not all of them. For example, in this pair, the methyl (CH3) and the alcohol (OH) kept the same configuration but not the bromine (Br). Diastereomers have different chemical and physical properties.

Summary
There are different ways to separate enantiomers. One way to do it is if one needs to separate a chiral alcohol, which is a molecule containing an oxygen atom linked to a carbon and an hydrogen, they can use a carboxylic acid that is either "S" or "R". Then, that person will create two diastereomers, and because they possess different physical and chemical properties, it is possible to separate them using various methods.

Ester
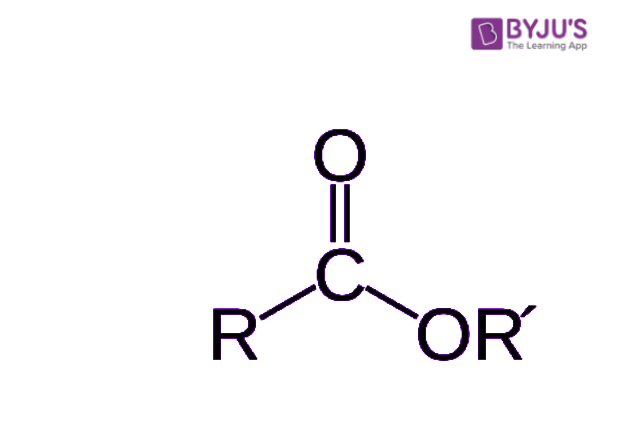
This process is called the "Mosher's esters" because, with the carboxylic acid, two esters are created. Another way of separating enantiomers is by using "chiral recognition". This method would use a reagent that wouldn't alter the structure of the enantiomers but still be able to separate them in only one step. However, this is only an ideal method, but in the source, it is said that it could be possible in a few years. There are other ways of separating racemic mixtures, but they come with many problems or are rarely successful. For example, there is chromatography that is, in most cases, ineffective, or kinetic resolution which is using the fact that some enantiomers may react more rapidly than others with another chiral molecule. Nevertheless, by using kinetic resolution, most of the time, the enantiomer that is the most reactive out of the two is altered permanently. Finally, even after the resolution, other methods must be used to determine which enantiomer someone is left with as well as its purity.
/GettyImages-545286316-433dd345105e4c6ebe4cdd8d2317fdaa.jpg)
My opinion
After reading about these resolution methods, I think that separating a racemic mixture is complex. In fact, I realized that some methods only apply to some types of molecules, but not to others. Also, some methods damage molecules so the yield is affected by it. To continue, it is also time-consuming because after someone has done the resolution, they need to analyze their final product to know which enantiomer they have, and to know if their product is pure. If this process is long, that also means that it is expensive because in a laboratory, time is money. On top of that, a low yield means a loss of money. So, overall, the resolution of racemic mixtures is not beneficial because it costs a lot of money, it is complicated, and it is a case-by-case process.

However, enantiomers are an important part of the pharmaceutical industry because they have different biological properties. In fact, a molecule can have a positive impact on a person's body while its enantiomer can be dangerous. For example, in the source, Thalidomide is mentioned. As a matter of fact, in the 1950s, a company started to sell the racemic mixture of Thalidomide, and one of the enantiomers was a sedative while the other caused fetal mutations.
Since enantiomers' effects can be dramatic, and according to the source, separating a racemic mixture isn't profitable because of its complexity and cost, I think that the best way of dealing with enantiomers is to avoid racemic mixtures. In fact, by synthesizing only one enantiomer, a person already has a pure product, and doesn't have to deal with all of these problems. I also think that if a chiral molecule used in a drug has an inactive enantiomer, the racemic mixture of the molecule could be commercialized for a drug because it wouldn't have any negative effects on people's health.

My question
Where did Henry Stone Mosher develop the "Mosher's esters" technique?
Sources for the images
Lactic acid:
https://cdn1.byjus.com/wp-content/uploads/2021/03/Examples-of-Enantiomers-01-700x261.png
Diastereomers:
https://i.ytimg.com/vi/23-dfkwKCcg/maxresdefault.jpg
Carboxylic acid:
Ester:
https://cdn1.byjus.com/wp-content/uploads/2018/07/Ester.png
Chemistry:
Expensive:
Complicated:
https://www.ouinolanguages.com/thumb/Italian/vocab/92/images/pic15.jpg
Thalidomide:
https://qph.cf2.quoracdn.net/main-qimg-b6df44f9bdd79c6f8d34401f491909fb
Organic chemist:
https://blog.cuw.edu/wp-content/uploads/andrea.jpeg
Hello, my name is Claudie Ouellet. I'll be commenting on your blog!
RépondreSupprimerHenry Stone Moscher pioneered the "moster's ester's" technique in Stanford University. The source Rémi used is scientific. First of all, I've never done chemistry in my life so bare with me, the resolution of enantiomers and racemic mixtures is extremely complex. To dissolve racemic bases, you need chiral acids, that will seperate the solution. There, it becomes an enantiomer, another form of the molecule. It has the same atoms, but attached differently to it and it reflects light in the other reflection that the original molecule does. I agree with Rémi that all this is very complex, and that the cost of doing this isn't worth the results. I have a sister who works in a laboratory and executes things of the kind, and I know that you can waste lots of molecules and products by trying these type of things. Since there are other, simpler ways of getting better result, I think it would be suitable to use those instead. Overall, chemistry is fascinating and requires a lot of thinking, so props to Rémi for learning more about the subject. For a first time doing anything chemistry related, I learned quite a few things!
RépondreSupprimer