Rémi Ouellette Blog #3
KETO-ENOL TAUTOMERISM
Hello! It's Rémi again! For this blog post, I decided to write about a chemistry-related subject again. So, I chose one that was related to a reaction we saw in class recently. This blog post will be about keto-enol tautomerism.

My source for this blog:
Important organic chemistry vocabulary:
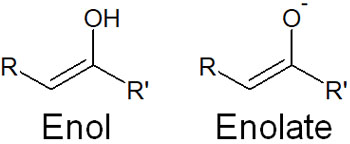
- Enol: An enol is a moiety containing an alcohol (OH) linked to a carbon-carbon double bond.
- Ketone: A ketone is a moiety that consists of a carbon atom linked with a double bond to an oxygen atom and also to two other carbon chains.
- Tautomerism: Tautomerism, as seen in the previous image, is when a molecule can change between two different forms, simply by rearranging its atoms. These two molecules usually have different levels of stability. So, the more stable form, which is usually the keto form, is more common.
- Tautomers: Tautomers are the two different forms of a molecule that does tautomerism.
- Enolate ion: The enolate ion is an enol that is missing the hydrogen that pertains to the oxygen atom. It is its conjugated base.
- Oxonium ion: The oxonium ion is a ketone with a hydrogen atom linked to its oxygen atom. It is its conjugated acid.
- Resonance: Resonance is a phenomenon that happens when electrons can be relocated on more than one atom. It makes molecules more stable.

Summary
To begin, as it was mentioned before, ketones are usually more stable than their enol equivalent because the carbon-oxygen double bond is stronger than the carbon-carbon double bond. Also, there are two carbon chains that "give" electrons to the carbon atom that is linked to the oxygen one, making it more stable. So, this means that an aldehyde's enol form is less stable than a ketone's enol form because only one carbon chain is "giving" electrons to the carbon when the moiety is an aldehyde.
Aldehyde and ketone: "R" represents whatever carbon chain
Furthermore, sometimes the enol form is more stable than the keto form. For example, in the case of 1,3-dicarbonyls, which is when two ketones are separated by one carbon atom, the enol is more stable than the ketone because in the first one, a hydrogen bonding is created and there is also resonance. Both of these are stabilizing for the molecule, making it more common.
Hydrogen bonding in the enol form
Resonance in 4-hydroxy-3-penten-2-one
Another example of resonance that is stabilizing for enols is in phenol. Because it is an aromatic molecule, it does a lot of resonance. Therefore, phenol is the only tautomer, and its keto form isn't frequent in nature.
Phenol
However, the forms' stability isn't an obstacle for a reaction because there are ways to go from one form to the other, usually by creating an enolate ion or an oxonium ion.
My opinion
After reading this source, I think that before doing a chemical reaction that involves creating a ketone or an enol, chemists must think about many aspects. In fact, as it is said in the source, ketones are usually the more stable tautomer, but there are exceptions. For example, if a chemist wants to create pentane-2,4-dione like in the previous picture, they must know that they will have to do extra steps because 85 percent of it will be rearranged in the enol form.

Also, because these two forms are present, I think that it can affect the yield of the reaction and result in a loss of money because if an industry only wants one form of the molecule, the remainder will not be useful and extra steps, and extra money, will be needed to transform it, or it will be thrown away, which is also a loss of money.

On another note, I think that tautomerism is important to humans because it takes place in our bodies. In fact, according to the source, an enzyme from the isomerase family turns ketose to aldose and an intermediate of this reaction is an ene-diol, an enol.
So, chemistry is really important to us. On top of that, in the source, many different methods to go from one form to the other are shown. There is, in fact, mechanisms to do it under basic conditions and others for acidic conditions. So, if a chemist wants to proceed with a tautomerization process, they need to choose which conditions to do it in based on the moieties of the molecule they want to change. I think that this is good because if the person wants to leave other moieties untouched, and these react in acidic conditions, they could do the reaction in basic conditions. But overall, a lot of thinking must be done when doing chemical reactions.
My question:
Which greek letter is given to the carbon(s) next to the one in the carbonyl group?
Sources for the images:
Keto-enol equilibrium:
Enolate:
Oxonium:
Aldehyde and ketone:
aldehyde_and_ketone_compare.jpg
Hydrogen bonding:
Resonance:
Example_2%2C4-pentanediol_enol.svg
Phenol:
chemical-structure-cas-108-95-2.jpg-650.jpg
Angry chemist:
funny-crazy-chemist-doing-experiments-tests-183379028.jpg
Money in the trash:
360_F_124789172_4gJDBl8ss2UtFSOF6H6jW8XJhQWfZhTU.jpg
Ketose to aldose reaction:
Commentaires
Enregistrer un commentaire